Transparency, Comprehension, and Compliance: Key Factors in Translating and Localizing Lay Summaries (LS)
Table of Contents
Providing lay summaries in plain language is a global trend to facilitate comprehension and transparency around clinical trial results. Since the EU Clinical Trial Regulation (CTR) 536/2014 requirement took full effect in January 2022, EU sponsors must provide lay summaries for all clinical trials (CT), regardless of phase or outcome. In the US, the FDA has been considering a similar initiative. And a growing number of scientific journals now expect or encourage jargon-free summaries of research articles for lay audiences.
These summaries provide benefits that range from improved accessibility, enhanced inclusion, and improved patient safety to more informed decision-making. But what happens when these documents require translation into multiple languages and variants? Why is partnering with an experienced, specialized language service provider (LSP) crucial to ensure successful dissemination across languages and cultures?
Reviewing the Basics: What are Lay Summaries (LS)?
As the name indicates, Lay Summaries summarize the results of clinical trials in a way that patients and the general public can easily understand, in a sense “translating” complex scientific content into reader-friendly language. They use plain language and appropriate principles of numeracy and design to provide consumer-friendly information on the objectives and the design of clinical trials. In addition, these documents explain risks, benefits, study endpoints, key findings, and outcomes. Lay Summaries are indispensable tools for patient and caregiver communication that enhance transparency and comprehension. Well-crafted summaries bridge the gap between scientific rigor and patient understanding, benefiting the research community and the public.
What about PLS and PLS-P?
Although there is some overlap in terminology, Lay Summaries (LS), Plain Language Summaries (PLS), and Plain Language Summaries for Publications (PLS-P) are distinct concepts.
Plain Language Summaries (PLS)
PLS can refer to two things. On the one hand, this is the term often used in the US to refer to LS, for example, when discussing whether the FDA plans to follow in the footsteps of the EMA and require these summaries for clinical trials. In terms of academic publications, the term refers to a summary in non-technical language, often around 250-300 words in length, submitted along with an academic abstract of a research article for publication, sometimes called a “lay abstract.”
Plain Language Summaries for Publications (PLS-P)
PLS-Ps are more extended forms of PLS in the context of peer-reviewed journals that fully summarize a published article in approachable, non-technical language. A PLS-P often includes images and illustrations and may be published in an infographic style, alongside the corresponding article, or as a standalone publication.
EU CTR Lay Summaries: Enhancing Transparency and Accessibility
While it fits the Patient-focused drug development initiative of the FDA, that authority has not yet taken steps to require clinical study results to be provided in plain language. The EMA has taken a different tack: The EU CTR explicitly stipulates that LS be provided and uploaded to the newly created Clinical Trials Information System (CTIS) for all beginning clinical trials. By January 31, 2025, ongoing trials must also have been transferred to CTIS.
Disseminating and publishing Lay Summaries on the CTIS supports several key objectives:
Patient safety and empowerment:
- Lay Summaries allow patients, families, and caregivers to access information about CT in a language and format they can understand.
- Lay Summaries empower patients to understand the potential risks, benefits, and outcomes of CTs.
Informed decision-making:
- Lay Summaries give patients and caregivers access to the information they need to make better decisions regarding their health, including participation in clinical trials.
Accessibility and inclusivity:
- Lay Summaries ensure that the results of CTs are accessible to the broadest possible audience – not only patients and caregivers but also the general public.
- Lay Summaries promote inclusivity by breaking down barriers relating to complex terminology, technical jargon, and literacy.
It is only logical that providing fair and equitable access to this content requires making it available in the native language of a given patient.
Language Access and Translation
According to Good Lay Summary Practice (GLSP), at a minimum, LS should be made available in the official local language or languages of each country where a trial was conducted. These languages should match those used in Patient Information Sheets (PIS) and Informed Consent Forms (ICF). If not authored in English, preparing an English version should also be considered to enhance accessibility throughout the EU and worldwide. GLSP also stipulates that these translated versions be provided as early as possible – ideally in parallel to the release of the original LS to ensure fair access to information for patients and the general public.
When conducting multi-country trials, translation is an integral part of the process that requires careful planning. Care must be taken to ensure that the meaning of the source document is preserved and that the cultural validity of the language is taken into account. In addition, the non-promotional nature of the LS must be maintained.
To see why this is such a vital part of successfully conducting a clinical trial across locales, it helps to have an idea of the complexity of two processes: LS development and translation.
The Lay Summary Process Explained
As per the GLSP Handbook, LS development consists of four key steps:
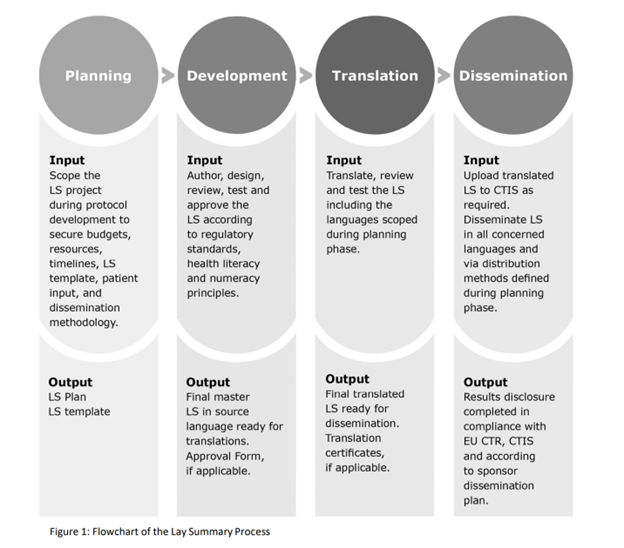
GSLP indicates the integral nature of the translation process to LS development and suggests how best to achieve the intended results.
Best Practices in Translating and Localizing LS
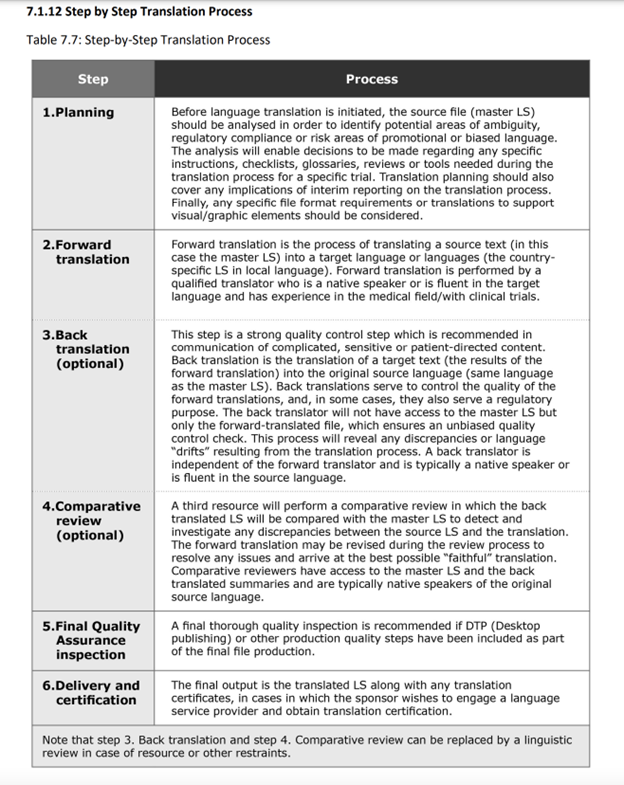
Helpfully, the Good Lay Summary Practice guidance, published in 2021, details the many steps required for quality translation output. It suggests the following process:
Your Language Services Partner: A Crucial Decision
LS translation is an intricate, resource-intensive process that requires deep expertise and experience and is a vital part of the success of a multi-country clinical trial. It cannot be overstated that while the translation and localization process occurs towards the end of the process, it cannot be an afterthought. GLSP explicitly states that “proactive planning and management will facilitate the quality, timeliness, and adequacy of LS to the target audience.”
This level of planning and process, especially when operating in such a highly regulated industry, can only be accomplished with the support of an experienced, specialized, and well-resourced language service provider.
Your language service provider should:
- Have extensive experience in the medical and clinical trial translation and localization space
- Have access to a roster of highly trained and specialized translators and localizers in all your required languages. These should be well-versed in the terminology and content of your therapeutic area, trained in plain language and numeracy principles, sensitive to potential cultural pitfalls, and have the requisite linguistic and scientific expertise.
- Employ a team of experienced project managers well versed in the industry and its processes.
- Have relevant glossaries, terminology databases, and style guides available.
- Ensure absolute confidentiality, data security, and adherence to GDPR.
- Be capable of ensuring compliance.
- Have processes in place to ensure:
-
- Accurate translation of complex medical terms and concepts
- Communication occurs at the correct reading level.
- Appropriate use of inclusive language.
- Avoidance of cultural pitfalls.
- Any potential issues are immediately flagged and resolved.
- No use of language that could be construed as promotional.
-
Lay Summaries Translation – A Summary
Lay summaries (LS) “translate” complex medical terminology and concepts into language patients and consumers can understand – a task that must done carefully to avoid losing meaning or accuracy. Making these available in multiple languages is a highly complex, intricate process that requires the expertise of an experienced life sciences localization team to ensure successful communication and cultural appropriateness.
As we have seen, a well-crafted LS is powerful: It empowers patients, facilitates informed decisions, increases access, promotes inclusivity, and – something increasingly important in today’s media landscape rife with mis- and disinformation – helps ensure that science is genuinely understood. Regulatory requirements aside, these benefits are far too significant to run the risk of having them get “lost in translation.”
If close collaboration with a well-resourced, experienced, and expert LSP is your goal, contact our team or see www.vistatec.com.

